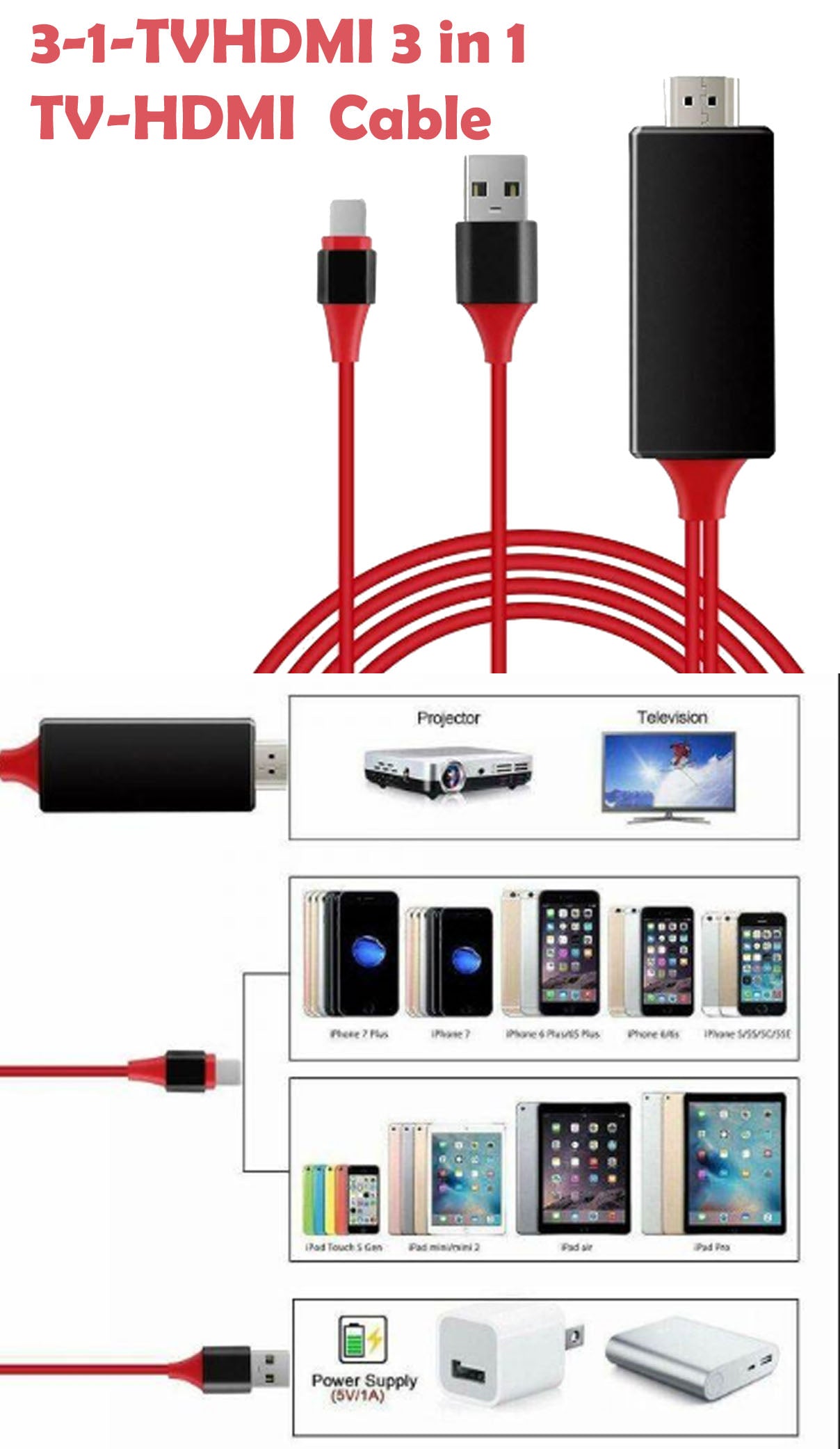
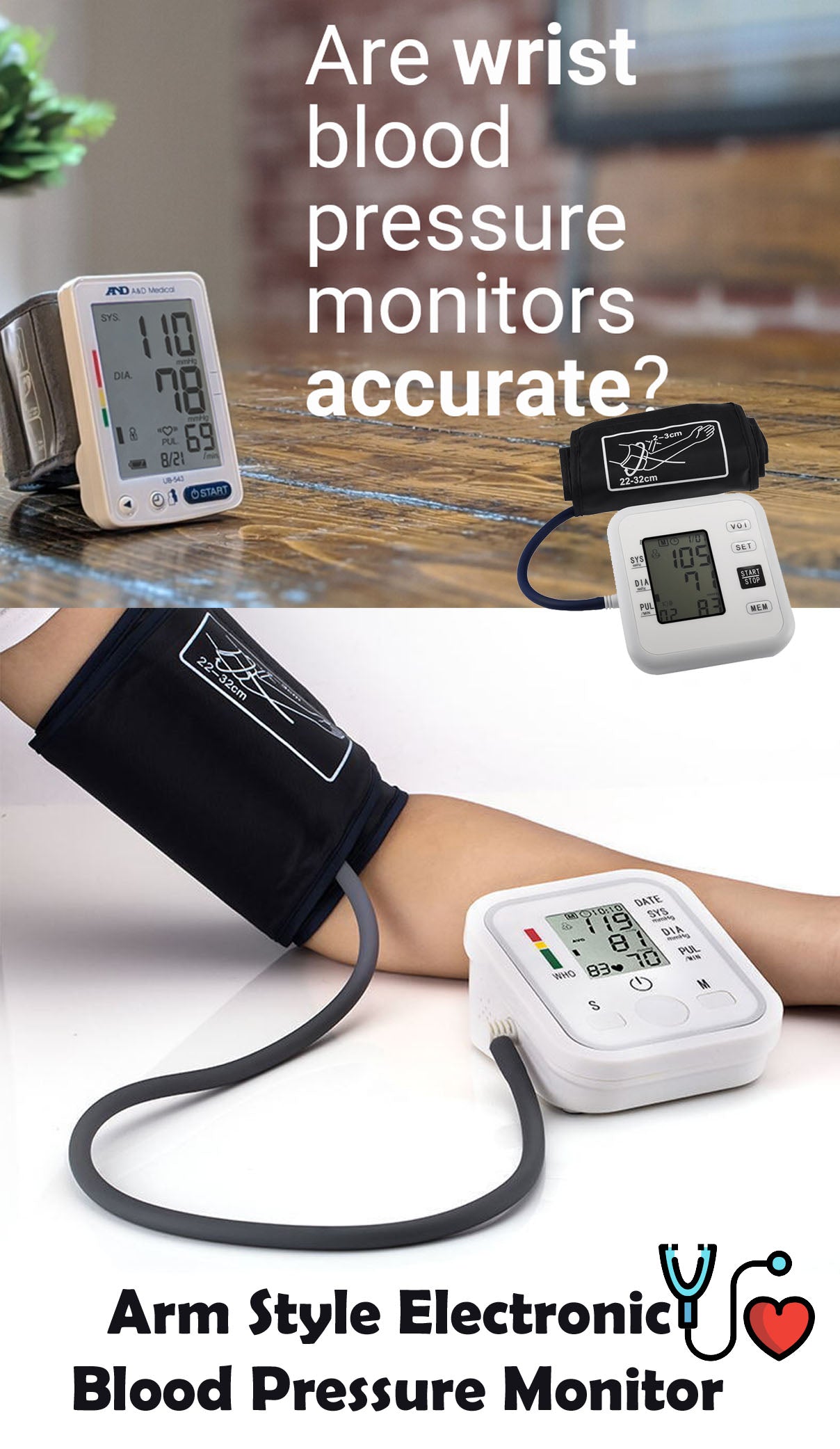
-30%


-
{"id":4561424613512,"title":"ZEBRA THRONE ARMCHAIR","handle":"zebra-throne-armchair","description":"\u003cp\u003e\u003cstrong\u003eZEBRA THRONE ARMCHAIR\u003c\/strong\u003e\u003c\/p\u003e\n\u003cp\u003eIf you’re a fan of our\u003cspan\u003e \u003c\/span\u003e\u003ca href=\"https:\/\/decorstore.com.au\/collections\/sofas-chairs\/products\/zebra-armless-chair\"\u003e\u003cspan\u003eZebra Armless Chair\u003c\/span\u003e\u003c\/a\u003e\u003cspan\u003e \u003c\/span\u003eor our\u003cspan\u003e \u003c\/span\u003e\u003ca href=\"https:\/\/decorstore.com.au\/collections\/sofas-chairs\/products\/zebra-lounge-chair?variant=12471848534065\"\u003e\u003cspan\u003eZebra Lounge Chair\u003c\/span\u003e\u003c\/a\u003e\u003cspan\u003e \u003c\/span\u003ethen you will appreciate our newest addition to the zebra Dazzle, our Regal Zebra Armchair. Alone, it is a fashionable sole addition in dressing rooms, bedrooms, and living spaces, as well as grouped around a dining table\u003c\/p\u003e\n\u003ch4\u003eSpecifications\u003c\/h4\u003e\n\u003cp\u003eSize: Height 100cm width 61cm Depth 56cm\u003c\/p\u003e\n\u003cp\u003eMaterials: 40% Polyester Fabric,40% Rubber Wood,15% Foam,5% Metal\u003c\/p\u003e","published_at":"2020-02-12T18:39:40+11:00","created_at":"2020-02-12T20:11:17+11:00","vendor":"Store Zone-Online Shopping Store Melbourne Australia","type":"ARMCHAIR","tags":["ARMCHAIR","Bedroom","Bedroom-Furniture","Decorstore All Products","Furniture"],"price":69500,"price_min":69500,"price_max":69500,"available":true,"price_varies":false,"compare_at_price":99900,"compare_at_price_min":99900,"compare_at_price_max":99900,"compare_at_price_varies":false,"variants":[{"id":32273401118856,"title":"Default Title","option1":"Default Title","option2":null,"option3":null,"sku":"DS","requires_shipping":true,"taxable":true,"featured_image":null,"available":true,"name":"ZEBRA THRONE ARMCHAIR","public_title":null,"options":["Default Title"],"price":69500,"weight":0,"compare_at_price":99900,"inventory_management":"shopify","barcode":"AV44667","requires_selling_plan":false,"selling_plan_allocations":[]}],"images":["\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_1_grande_grande_e47874b2-ec7b-407c-8e86-85302ccfa8b8.jpg?v=1581647820","\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_grande_grande_grande_732e6b07-241b-40ef-a615-987a04a536e6.jpg?v=1581647820","\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_2_grande_grande_ec778cb8-035d-4400-abcc-a1bbea31fe53.jpg?v=1581647820","\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_3_grande_grande_04bf6328-b2c9-42d8-91c4-cc3de7a76b8d.jpg?v=1581647820","\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_4_grande_grande_6c78561b-cada-4bfa-ac36-bed359eb1571.jpg?v=1581647820","\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_grande_grande_300x300_8b24acfa-b2c8-42d9-8097-bc4f392179b2.jpg?v=1581647820"],"featured_image":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_1_grande_grande_e47874b2-ec7b-407c-8e86-85302ccfa8b8.jpg?v=1581647820","options":["Title"],"media":[{"alt":"ZEBRA THRONE ARMCHAIR - Store Zone-Online Shopping Store Melbourne Australia","id":6654716280968,"position":1,"preview_image":{"aspect_ratio":1.0,"height":600,"width":600,"src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_1_grande_grande_e47874b2-ec7b-407c-8e86-85302ccfa8b8.jpg?v=1581647820"},"aspect_ratio":1.0,"height":600,"media_type":"image","src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_1_grande_grande_e47874b2-ec7b-407c-8e86-85302ccfa8b8.jpg?v=1581647820","width":600},{"alt":"ZEBRA THRONE ARMCHAIR - Store Zone-Online Shopping Store Melbourne Australia","id":6654716346504,"position":2,"preview_image":{"aspect_ratio":1.007,"height":596,"width":600,"src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_grande_grande_grande_732e6b07-241b-40ef-a615-987a04a536e6.jpg?v=1581647820"},"aspect_ratio":1.007,"height":596,"media_type":"image","src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_grande_grande_grande_732e6b07-241b-40ef-a615-987a04a536e6.jpg?v=1581647820","width":600},{"alt":"ZEBRA THRONE ARMCHAIR - Store Zone-Online Shopping Store Melbourne Australia","id":6654716379272,"position":3,"preview_image":{"aspect_ratio":1.0,"height":600,"width":600,"src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_2_grande_grande_ec778cb8-035d-4400-abcc-a1bbea31fe53.jpg?v=1581647820"},"aspect_ratio":1.0,"height":600,"media_type":"image","src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_2_grande_grande_ec778cb8-035d-4400-abcc-a1bbea31fe53.jpg?v=1581647820","width":600},{"alt":"ZEBRA THRONE ARMCHAIR - Store Zone-Online Shopping Store Melbourne Australia","id":6654716444808,"position":4,"preview_image":{"aspect_ratio":1.0,"height":600,"width":600,"src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_3_grande_grande_04bf6328-b2c9-42d8-91c4-cc3de7a76b8d.jpg?v=1581647820"},"aspect_ratio":1.0,"height":600,"media_type":"image","src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_3_grande_grande_04bf6328-b2c9-42d8-91c4-cc3de7a76b8d.jpg?v=1581647820","width":600},{"alt":"ZEBRA THRONE ARMCHAIR - Store Zone-Online Shopping Store Melbourne Australia","id":6654716477576,"position":5,"preview_image":{"aspect_ratio":1.0,"height":600,"width":600,"src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_4_grande_grande_6c78561b-cada-4bfa-ac36-bed359eb1571.jpg?v=1581647820"},"aspect_ratio":1.0,"height":600,"media_type":"image","src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_4_grande_grande_6c78561b-cada-4bfa-ac36-bed359eb1571.jpg?v=1581647820","width":600},{"alt":"ZEBRA THRONE ARMCHAIR - Store Zone-Online Shopping Store Melbourne Australia","id":6654716510344,"position":6,"preview_image":{"aspect_ratio":1.007,"height":298,"width":300,"src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_grande_grande_300x300_8b24acfa-b2c8-42d9-8097-bc4f392179b2.jpg?v=1581647820"},"aspect_ratio":1.007,"height":298,"media_type":"image","src":"\/\/storezone.com.au\/cdn\/shop\/products\/AV44667_grande_grande_300x300_8b24acfa-b2c8-42d9-8097-bc4f392179b2.jpg?v=1581647820","width":300}],"requires_selling_plan":false,"selling_plan_groups":[],"content":"\u003cp\u003e\u003cstrong\u003eZEBRA THRONE ARMCHAIR\u003c\/strong\u003e\u003c\/p\u003e\n\u003cp\u003eIf you’re a fan of our\u003cspan\u003e \u003c\/span\u003e\u003ca href=\"https:\/\/decorstore.com.au\/collections\/sofas-chairs\/products\/zebra-armless-chair\"\u003e\u003cspan\u003eZebra Armless Chair\u003c\/span\u003e\u003c\/a\u003e\u003cspan\u003e \u003c\/span\u003eor our\u003cspan\u003e \u003c\/span\u003e\u003ca href=\"https:\/\/decorstore.com.au\/collections\/sofas-chairs\/products\/zebra-lounge-chair?variant=12471848534065\"\u003e\u003cspan\u003eZebra Lounge Chair\u003c\/span\u003e\u003c\/a\u003e\u003cspan\u003e \u003c\/span\u003ethen you will appreciate our newest addition to the zebra Dazzle, our Regal Zebra Armchair. Alone, it is a fashionable sole addition in dressing rooms, bedrooms, and living spaces, as well as grouped around a dining table\u003c\/p\u003e\n\u003ch4\u003eSpecifications\u003c\/h4\u003e\n\u003cp\u003eSize: Height 100cm width 61cm Depth 56cm\u003c\/p\u003e\n\u003cp\u003eMaterials: 40% Polyester Fabric,40% Rubber Wood,15% Foam,5% Metal\u003c\/p\u003e"}